THIS ARTICLE IS MORE THAN FIVE YEARS OLD
This article is more than five years old. Autism research — and science in general — is constantly evolving, so older articles may contain information or theories that have been reevaluated since their original publication date.


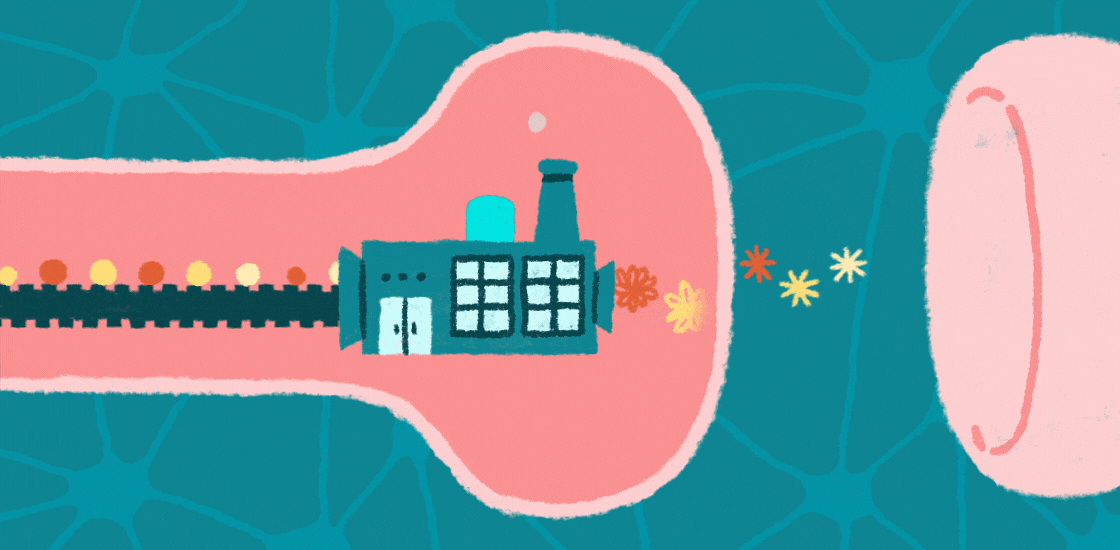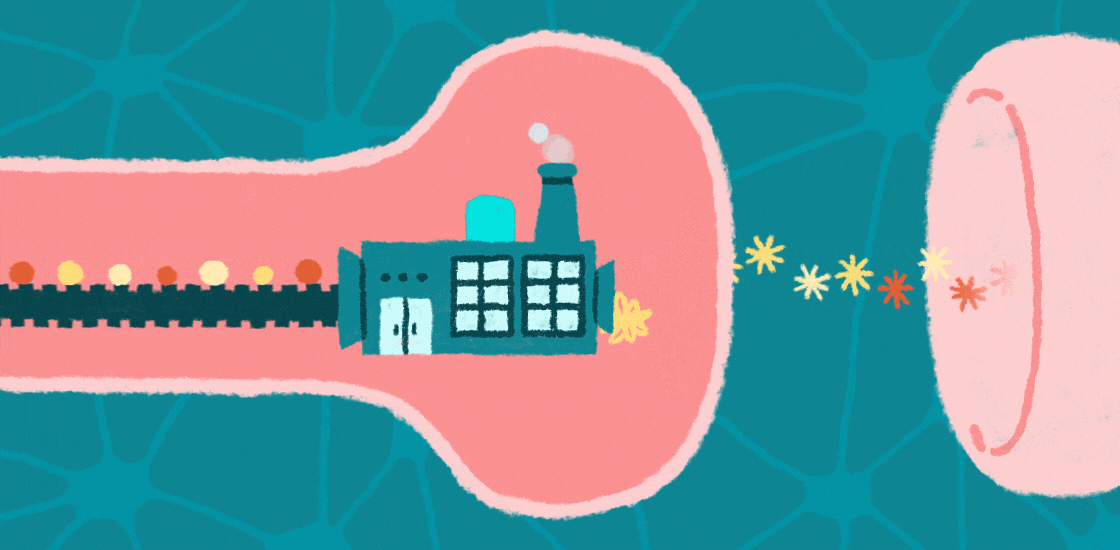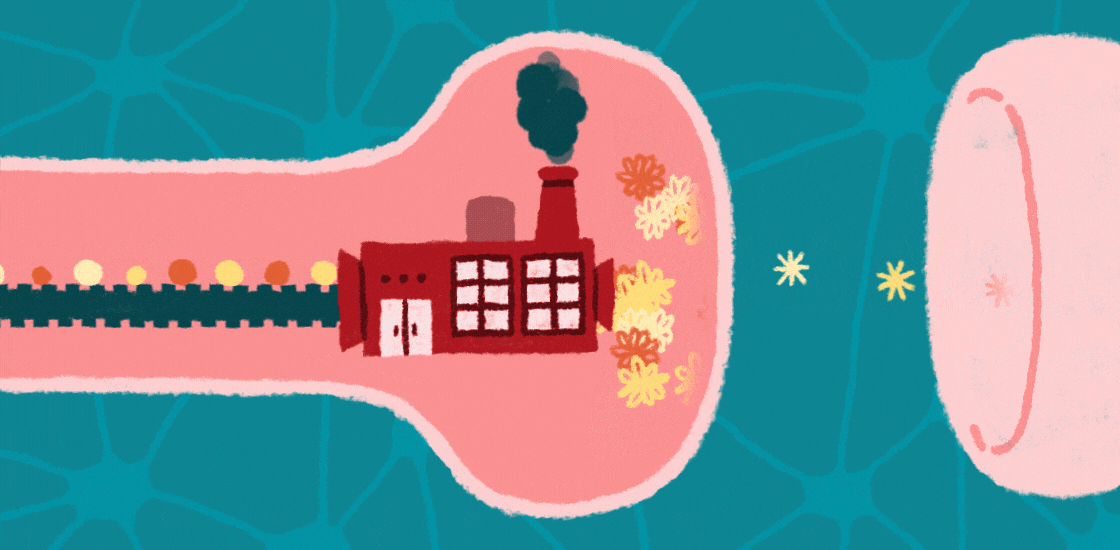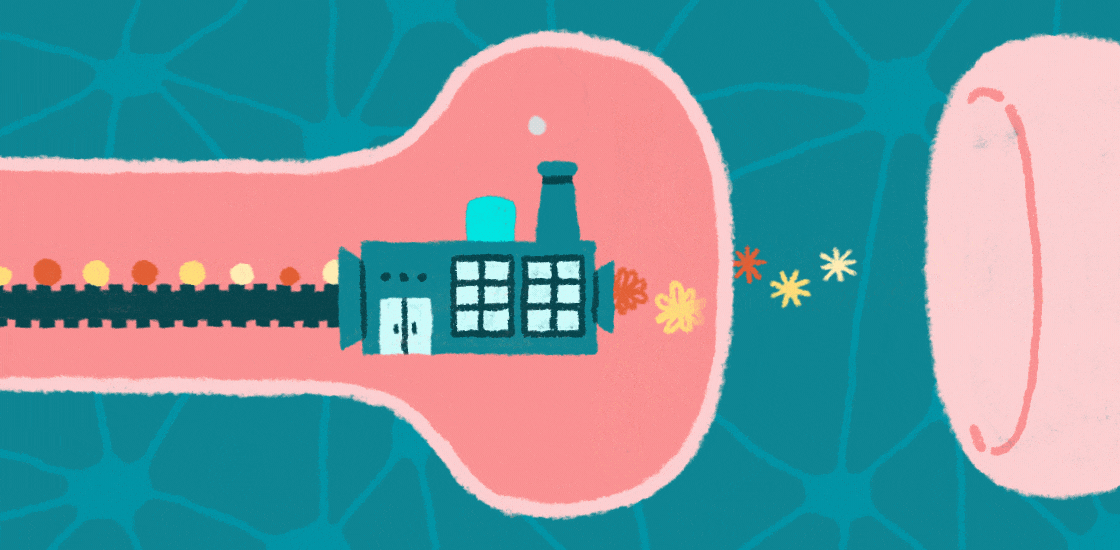
Learning, memory and social behaviors all hinge on the tight control of messages passed between brain cells at junctions called synapses. This communication depends on proteins at these synapses that allow neurons to respond appropriately to signals, such as developmental programs and environmental cues.
Increasing evidence suggests that autism is a ‘synaptopathy,’ a condition in which protein production is disrupted at synapses, leading to too many, or possibly too few, proteins at these junctions. Changes in the number or type of proteins at synapses can lead to problems with brain signaling, which could underlie features of autism.
Protein production relies on messenger RNA (mRNA), molecules that contain the information encoded in the genomic DNA. In the past few decades, researchers have identified mutations in genes that regulate the translation of mRNAs into proteins at synapses.
These mutations affect the structure of synapses and perturb the transmission of messages across them. They may also limit the ability of synapses to change their shape as a result of experience, a process called synaptic plasticity that underlies brain functions such as learning and memory. Interestingly, several of these mutations are associated with autism, suggesting that disrupted protein synthesis contributes to this condition.
Perturbed proteins:
One of the identified mutations causes fragile X syndrome; at least one-third of people with this syndrome are also diagnosed with autism1. This mutation prevents the production of the fragile X protein, FMRP. In the brain, FMRP binds CYFIP1 and EIF4E, forming a molecular complex at synapses that slows the translation of mRNAs into proteins that are important for synaptic plasticity, cognition and behavior2,3.
When FMRP is mutated or completely absent, neuronal and synaptic protein production surges and becomes unbalanced. Mutations in CYFIP1 and EIF4E also raise the risk of autism4.
Deficits upstream from FMRP activity and protein synthesis can also be relevant for autism. For example, mutations in the genes TSC1 or TSC2 — which are upstream from the protein synthesis machinery — cause an autism-related condition called tuberous sclerosis, in which benign tumors appear throughout the brain and body5. One of the hallmarks of this condition, at least in mouse models, is perturbed synaptic protein synthesis5,6.
Studies in cells of individuals with autism also show altered expression of genes in pathways that are important for protein synthesis in neuronal branches (dendrites and axons) that terminate at synapses7, 8, 9, 10. These results further support the involvement of dysfunctional synaptic proteins in autism.
Immune cells called microglia, which may be found near synapses, show increased activation in autism brains. Postmortem brain studies show that some genes controlling the activity of these microglia are dysregulated in people with autism11. These findings suggest that inflammation might also modulate local protein synthesis.
Targeting networks:
No gene operates alone. The encoded proteins contribute to biological processes requiring input from other proteins. These interactions generate vast networks around shared biological functions.
Autism-risk genes in non-synaptic pathways, such as those that govern how DNA is packaged, also are thought to have an impact on protein synthesis. Researchers are now beginning to focus their studies on identifying synaptic processes, as well as other biological processes, that could be targeted with drugs.
Although we are still unraveling the many ways these complex gene networks contribute to autism, it is becoming increasingly clear that the regulation of synaptic-protein production is a key factor —a biological process that underlies both intellectual disability and autism.
Several questions still need to be addressed. Do the different synaptic proteins act at a critical time in development, and when would treatment be most effective? Which brain regions are particularly susceptible to altered protein synthesis, and are some regions more relevant to autism than others?
Researchers also should investigate how molecular networks orchestrating protein synthesis interact with environmental factors such as toxins, infections and stress.
Our growing understanding of protein production at synapses is already providing clues to new therapies. Several ongoing clinical trials are targeting this mechanism in the hopes of improving the outcomes of people with autism.
Claudia Bagni is professor in the fundamental neurosciences department at Université de Lausanne in Switzerland. Fiona Hollis is Marie Heim-Vögtlin Fellow of fundamental neurosciences at the university.
By joining the discussion, you agree to our privacy policy.