THIS ARTICLE IS MORE THAN FIVE YEARS OLD
This article is more than five years old. Autism research — and science in general — is constantly evolving, so older articles may contain information or theories that have been reevaluated since their original publication date.

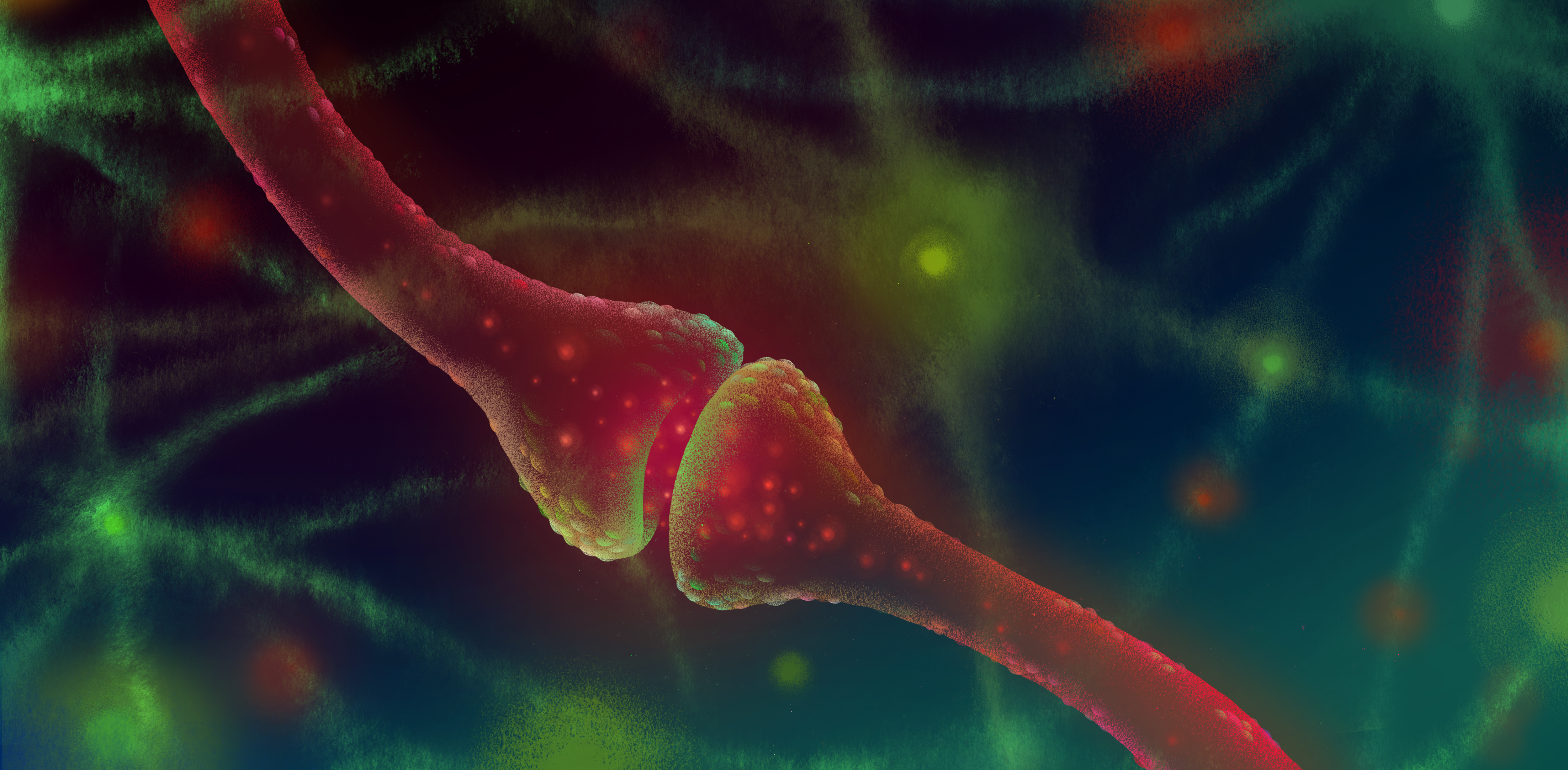
A chemical messenger that quiets brain activity has been a not-so-quiet player in autism. The messenger, known as gamma-aminobutyric acid (GABA), may be too scarce in people with the condition. The resulting signal imbalance may lead to a brain that is overactive — a state that could make people with autism prone to epilepsy or hypersensitive to stimuli such as light or sound.
A new report reveals another role for GABA, this one during development. While studying the formation of synapses, the connections between neurons, in newborn mice, researchers discovered that GABA directs the placement of synapses. The researchers were surprised to find that it affects both excitatory and inhibitory synapses, rather than only inhibitory ones.
The findings suggest that the effects of GABA deficiency run deep. Too little GABA could prevent synapses from forming in the right spots, in addition to shifting the signaling balance. The new study appeared 11 August in Science1.
We asked the senior investigator, Hyungbae Kwon, a research group leader at the Max Planck Florida Institute for Neuroscience, what the newly discovered role for GABA might mean for autism.
Spectrum: Did people know prior to your study that GABA has a role in forming synapses?
Hyungbae Kwon: Decades’ worth of studies show that GABA is important for synapse formation. But when you explore the role of GABA by mutating a gene, you create a global effect. So while you can conclude that GABA is important for synapse formation, you cannot say exactly what GABA is doing.
We can now use a technique called two-photon uncaging to study this question in individual synapses. This allows us to define how a single synapse forms and to see how GABA directs this formation.
S: How does this tool work?
HK: It’s based on two-photon microscopy, which delivers short laser pulses into a tissue. This lets us look at fine structures such as synapses in living tissue without damaging the tissue.
Two-photon imaging can be combined with a process called uncaging that we use in our cell cultures, which mimic mouse brain tissue. Basically, we deliver a synthetic neurotransmitter that mimics GABA or glutamate but is inactive because it’s linked to a caging compound. When we direct light from a laser to a certain site on the cell surface, the neurotransmitter there loses the caging compound and becomes active.
In a Nature paper a few years ago, we used two-photon uncaging to release glutamate anywhere we wanted, at any time we wanted, in tissue cultures of mouse brain tissue2. We created patterns of glutamate release and looked at the effects on young neurons. We found that glutamate release is sufficient to induce a new excitatory synapse but not an inhibitory synapse.
The obvious next question was: What happens if you release GABA at a synapse? So in our new study, we used the same technique to release GABA at precise locations on cultured mouse neurons, both immature and mature ones.
S: What did you find?
HK: We found that most synapses — both excitatory and inhibitory ones — formed within a two-micron window from the GABA release site in immature neurons. So GABA actually plays an important role in forming both types of synapses.
This is surprising because GABA is known as an inhibitory neurotransmitter. Its major function is to quiet things down when the brain gets too excited. So most people thought that GABA just induces inhibitory synapses and transmits inhibitory signals, while the excitatory neurotransmitter glutamate induces excitatory synapses.
Before this study, no one knew why synapses are made where they are. We now think that the axons, or long projections, of GABA neurons grow toward a target area. When the axonal terminal, which is the GABA release site, gets close enough to this target area, it starts to release GABA. The target neurons sense this GABA release, and that is a critical first step for synapse formation.
We think GABA may create some kind of hotspot on the neuron — an area that is somehow more suitable for synapse formation. But this is just speculation. Further studies will explore this hypothesis.
S: What might happen when this process goes awry?
HK: The location of a synapse determines how a neuron computes information. If the spatial distribution of inhibitory or excitatory synapses is wrong, the integration of information goes wrong. That can impair a neuron’s role in, say, memory or learning.
It’s interesting that autism symptoms usually appear sometime between birth and kindergarten —the same period that the majority of synapses are formed for life. We know that many autism-related genes — such as the members of the SHANK and neuroligin families — encode proteins that are important for synapse formation. So it’s possible that the target areas for synapse formation are altered in people with autism.
By joining the discussion, you agree to our privacy policy.