THIS ARTICLE IS MORE THAN FIVE YEARS OLD
This article is more than five years old. Autism research — and science in general — is constantly evolving, so older articles may contain information or theories that have been reevaluated since their original publication date.
The popular gene-editing tool CRISPR can tag proteins in the brain, illuminating the whereabouts of autism candidates, suggests a new study1.
Traditional tools for tracking proteins typically involve labeling a protein using sticky immune molecules, called antibodies, that fluoresce. But the method is imprecise because some antibodies stick to many proteins. Scientists can make mice that produce the protein of interest with a fluorescent tag attached, but doing so requires breeding animals, a time-consuming process.
The new method, described 16 June in Cell, is both rapid and reliable. It harnesses CRISPR, a tool involving a scissor-like enzyme that cuts through both DNA strands at precise locations. The researchers add a customized strip of DNA that the cell’s repair machinery inserts into the genome as it tries to rejoin the severed strands — attaching a DNA tag to a gene of interest.
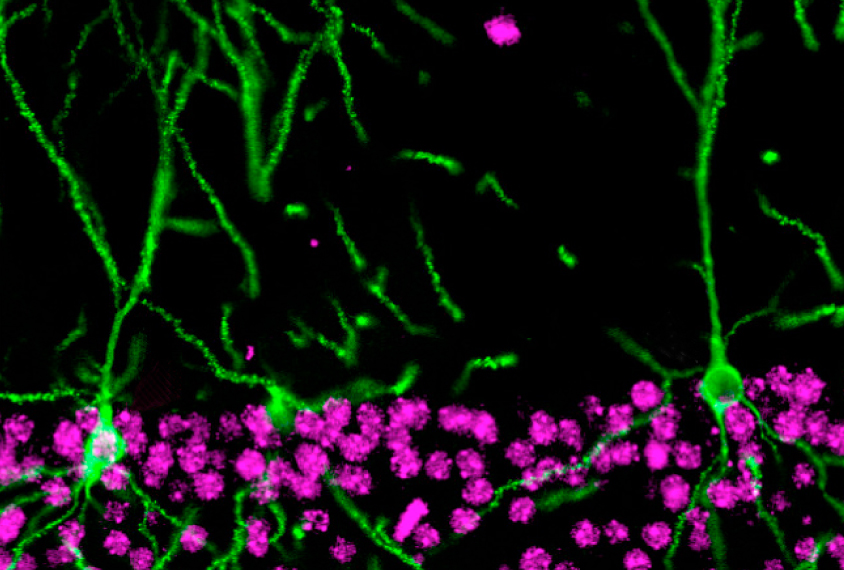
Proteins produced from the modified gene carry this custom tag, which can be visualized in brain tissue samples using fluorescent antibodies that stick to the tag.
Because this form of repair tends to occur only when cells divide, the researchers introduced the CRISPR machinery into the brains of mouse embryos, in which neurons are still dividing.
Mark and erase:
They used the technique, dubbed SLENDR, to see a variety of proteins, one at a time, in brain tissue samples taken from mice of various ages. In one test, the researchers spotted FMRP — the protein missing in people with fragile X syndrome — accumulating in discrete spots outside the nucleus of neurons.
The new technique is versatile. Applying SLENDR at various times and locations in the developing brain allows the researchers to tag proteins in different cell types and brain regions of adult mice.
The researchers also modified the method to track proteins in brain slices growing in culture: They inserted the sequence for a green fluorescent protein, which doesn’t require staining with antibodies.
SLENDR can also simultaneously mark one protein and delete the gene for another. (Gene deletion is a standard CRISPR task.) For example, labeling actin, a cell skeleton protein, and deleting MeCP2, the gene mutated in Rett syndrome, reveals that MeCP2 loss leads to a reduction of neuronal extensions called dendritic spines.
By joining the discussion, you agree to our privacy policy.