THIS ARTICLE IS MORE THAN FIVE YEARS OLD
This article is more than five years old. Autism research — and science in general — is constantly evolving, so older articles may contain information or theories that have been reevaluated since their original publication date.
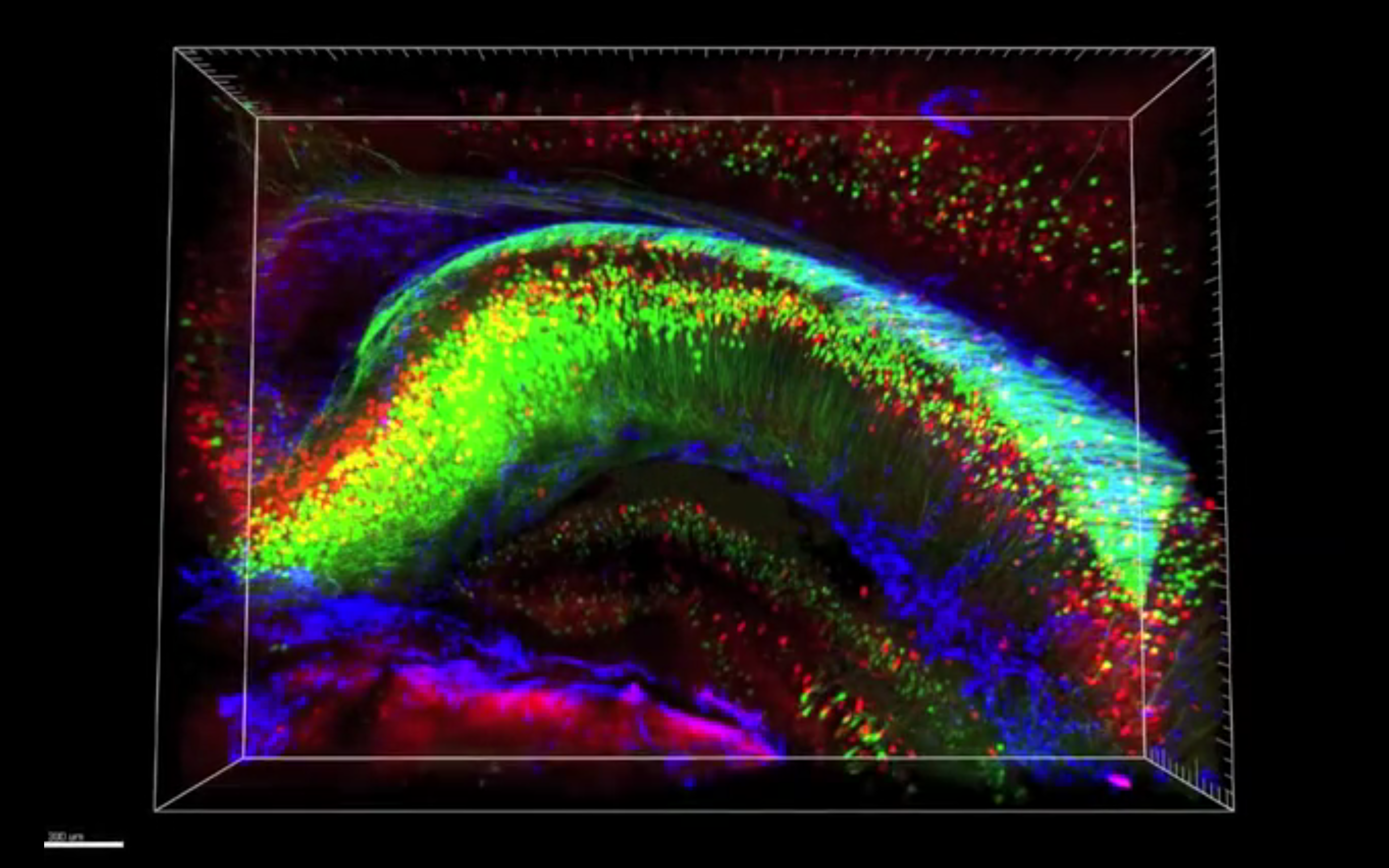
A new method that renders the brain transparent generates unprecedented views of long-range neuronal connections, researchers report today in Nature1.
“It was an emotional experience to see brain circuitry revealed in so much detail with so much depth resolution,” says Samuel Wang, associate professor of neuroscience at Princeton University, who is working with the researchers to use the method in his own lab.
To photograph a whole brain using traditional imaging methods, researchers must first slice it into thousands of sections and photograph each one. This approach is not only tedious, but it severs brain connections that may not realign correctly when the images are assembled, says lead investigator Karl Deisseroth, professor of bioengineering at Stanford University in California.
| Click on the dots to rotate through videos. Footage courtesy of K. Chung and K. Deisseroth, Stanford U. |
In the new technique, called CLARITY, researchers remove the fat molecules, or lipids, that scatter light as it travels through the brain, while still maintaining the brain’s structure. This allows them to photograph an intact mouse brain by taking only two images, one from the top and one from the bottom.
Because it preserves long-range connections between brain regions, CLARITY is ideal for studying neurological disorders such as autism that may result from abnormal brain connections, researchers say.
“We always say we want to look at connectivity problems, but [until now] we didn’t have good tools to say what these problems are,” says Guoping Feng, professor of brain and cognitive sciences at the Massachusetts Institute of Technology, who was not involved in the study.
Most research on CLARITY has focused on mouse brains, but it can also be used to study postmortem human tissue. As proof of principle, Deisseroth’s team has already used the technique to analyze tissue from the postmortem brain of someone who had autism.
Fat loss:
One of the strengths of the technique is that it allows researchers to apply multiple markers to stain different sets of neurons. They can photograph the brain after each round of staining, which can include up to four antibodies, and then wash them away, allowing for multiple rounds of staining.
Overlaying the resulting images creates a picture of a brain brightly painted with layer upon layer of information, mapping out different types of neurons and their interrelated networks.
Video reconstructions of these maps have wowed audiences since Deisseroth first presented the technique at the 2012 Society for Neuroscience Annual Meeting in New Orleans.
“We started whispering to one another during the talk and the whispering just didn’t stop,” says Wang, who saw the videos at Princeton in January.
The technical advance that makes CLARITY possible comes from applying a chemical engineering approach to a biological problem, says Deisseroth. The problem is that the lipids that encircle cells block light and keep out antibodies. But they also provide the structural support that keeps the brain intact.
Deisseroth’s team uses two sets of chemical compounds to address this hurdle. One of these chemicals, formaldehyde, links proteins and other molecules that are in close physical contact in the cell, preserving those interactions. The others are hydrogels — water-absorbent flexible gels — including acrylamide, which is commonly used in labs to separate proteins. Formaldehyde also attaches the hydrogels to proteins within the brain.
Heating brains saturated in these chemicals triggers a similar gel to form within the brain, “building an infrastructure from within,” says Deisseroth. The gel binds to proteins, DNA and RNA but not to lipids, allowing researchers to expel the lipids out of the brain using detergents and an electrical current.
Once the brain is clear of the fat molecules, antibodies and other staining molecules travel easily through the gel and access their target proteins. Because of the gel matrix, the brain is able to weather the harsh detergents used to wash out each round of antibody staining.
Early adoption:
Researchers see a multitude of possibilities for applying CLARITY to studying autism. Because all the neurons in one brain can be labeled at the same time, researchers say, they will be better able to quantify the numbers and types of neurons in different brain regions without introducing variability between experiments.
Deisseroth’s team is applying this technique to various mouse models of neurological disorders, including mice lacking the CNTNAP2 gene, which has been linked to autism.
Imaging studies suggest that people who have a certain variant in CNTNAP2 have weak connections between distant brain regions. Analyzing brain anatomy in mice in detail could provide a much clearer picture of the variant’s effect on connectivity.
Researchers can also pair CLARITY with other techniques, to see how neuronal activity affects brain connectivity. For example, they can use molecules that light up when neurons fire to look at brain activity in live mice. A CLARITY analysis on these same brains can show how this activity influences overall brain connectivity, says Feng.
Preliminary findings from the human postmortem brain suggest that there are abnormalities in a type of neuron that inhibits brain signals. However, this could be an artifact, because the brain sat in solution for years, cautions Wang. “As with any new technique, we still have to apply all the old cautions and controls,” he says.
Though not labor intensive, CLARITY is time consuming. It takes nearly six weeks for one round of antibodies to diffuse through an entire mouse brain — and presumably longer for a human brain. In some cases it may be more efficient to cut the brain into chunks, says Deisseroth.
Photographing the stained brains using existing microscopy methods — which zoom incrementally into the brain, photographing different sections — could take months, says Wang. The imaging technology will need to catch up to CLARITY, he says.
Still, this isn’t stopping research teams from eagerly anticipating their own glimpse inside their favorite brain pathway. “A third of my email right now is about how I might be able to apply this technique,” says Wang. “We want to be one of the first groups to use it.”
By joining the discussion, you agree to our privacy policy.