THIS ARTICLE IS MORE THAN FIVE YEARS OLD
This article is more than five years old. Autism research — and science in general — is constantly evolving, so older articles may contain information or theories that have been reevaluated since their original publication date.
Editor’s Note
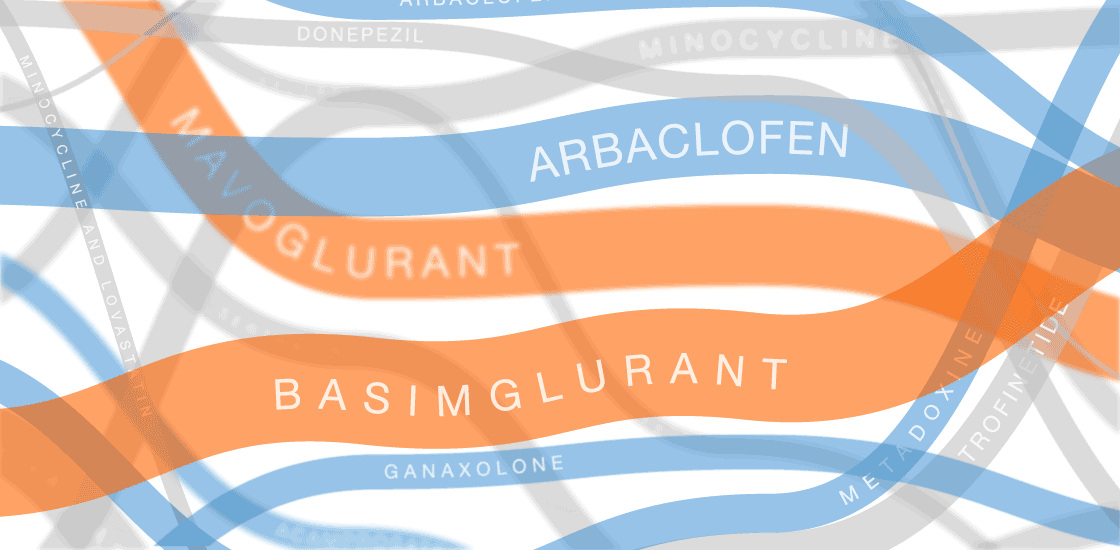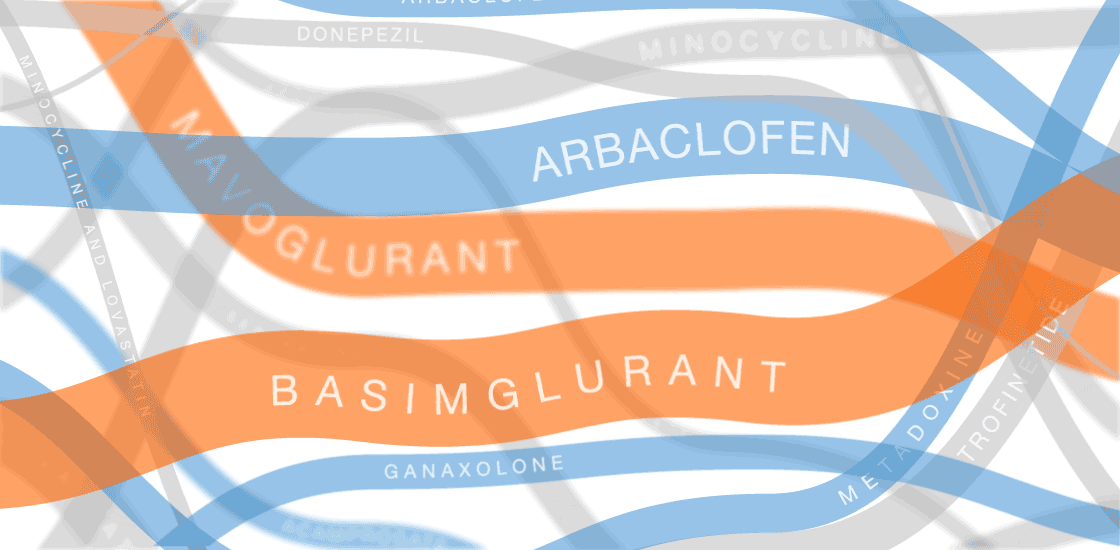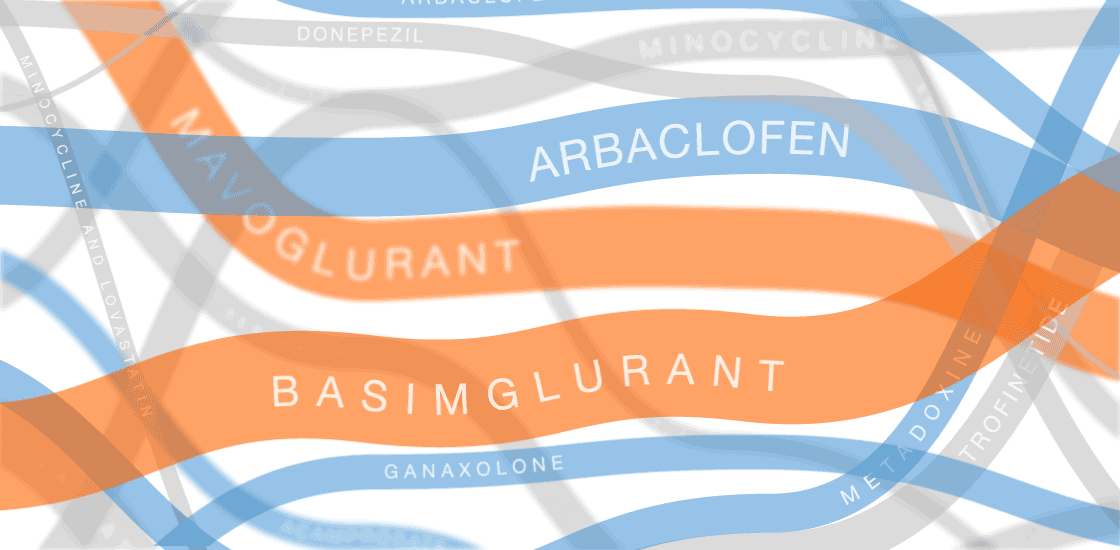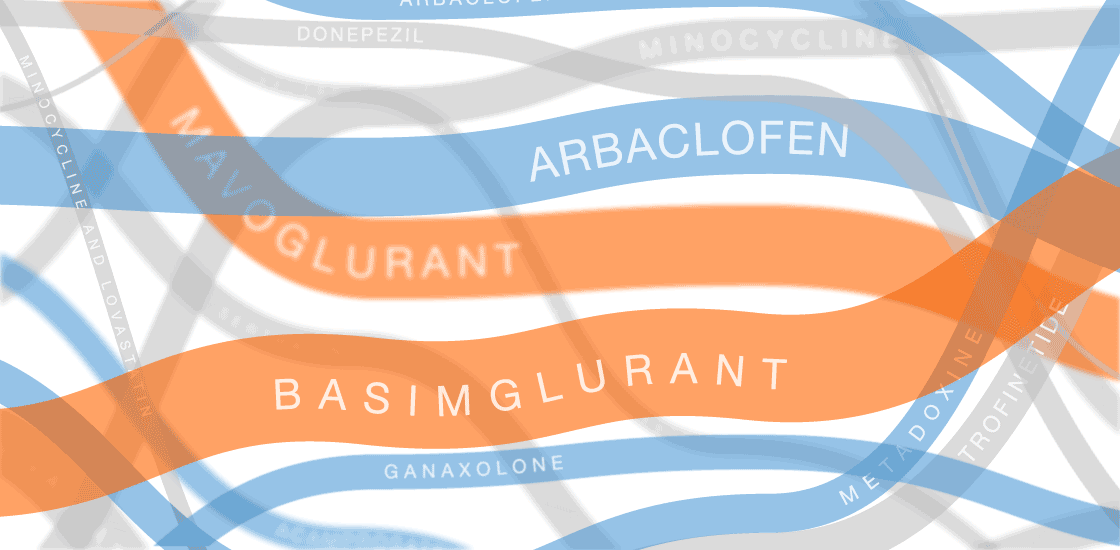
The graphic in this article was updated on 8 August 2017 to include additional clinical trials and new information for ongoing trials.
The road to treatments for fragile X syndrome is paved with solid facts and sound premises. But the syndrome — characterized by intellectual disability, hyperactivity, seizures and, often, autism — has so far resisted a pharmaceutical solution.
In the past several years, multiple fragile X treatments have undergone vetting in clinical trials1. So far, only one trial has shown improvement on the primary outcome measure — in that case, a clinician’s overall impression — but it was too small to be conclusive. Other trials are ongoing.
Fragile X syndrome stems from a mutation in a single gene called FMR1. This genetic cause distinguishes the syndrome from many other forms of autism, which typically have multiple genetic contributors. In theory, this genetic specificity should steer researchers toward effective treatments.
Yet FMR1 turns out to be the tip of the molecular iceberg underlying the condition: Its protein regulates thousands of other proteins in the cell. As a result, it is unclear what cellular pathways lead to the condition’s features.
Several of the treatments for fragile X syndrome block a receptor called mGluR5.
These treatments interfere with a pathway that stimulates excitatory signaling in the brain. Other candidates boost inhibitory signaling through a molecule known as gamma-aminobutyric acid (GABA) as a way to counteract excess excitatory activity.
Crystal ball:
These therapies have worked well in mouse models of the condition, but the mice don’t accurately mirror the condition in people. For example, mice lacking FMRI fail to show the cognitive deficits that people with fragile X syndrome do. As a result, researchers have had trouble determining which fragile X features to measure when they move a treatment into human trials.
In designing a trial, scientists need to specify a particular feature or ‘primary endpoint’ they aim to ameliorate. If this feature remains unmoved, the drug is unlikely to be approved — even if it eases other features of the condition.
So far, no treatment for fragile X syndrome has made a significant dent in its primary endpoint, although in some cases, a drug has improved other aspects of the syndrome.
Researchers have not given up, however. They are working on better ways to test the effects of drugs on people. They are also trying drug combinations and drugs paired with behavioral therapy.
By joining the discussion, you agree to our privacy policy.