Spectrum: Autism Research News
The gene hunters
Criss-crossing the globe on a quest for unusual DNA, researchers have discovered a rare mutation that promises insights into both epilepsy and autism — and points to a treatment.

Five years ago, pediatric neurologist Joseph Gleeson reached out to a doctor in Libya, seeking assistance with some scientific sleuthing. Gleeson’s research team at the University of California, San Diego was amassing a genetic database of children in the Middle East who have inherited brain conditions. The researchers had a strong hunch that they had uncovered a genetic culprit that could instigate not only autism, but also epilepsy and intellectual disability, conditions that frequently occur alongside autism. In this database, they had just come across a large Libyan family whose members share this rare DNA defect.
Gleeson had long thought that the puzzling overlap between autism and epilepsy must be important. Roughly 20 to 30 percent of people who have autism also have seizures, and as much as 20 percent of those with epilepsy also have autism. One possible explanation for this overlap is that the same underlying phenomenon gives rise to both conditions. Gleeson reasoned that studying children within this intersection of troubles could offer opportunities for finding some of the faulty genes — and, perhaps, for developing treatments.
His search led him to the rare mutations, which were in a gene called BCKDK. Before learning of the Libyan family, Gleeson’s crew had known of only two other families in the world with this DNA glitch, one of which had been identified by competing scientists at Yale University. The clan in Libya included two affected boys who were patients of neurologist Majdi Kara at Tripoli Children’s Hospital. In a phone call with the physician, Gleeson explained that the mutations could be fueling metabolic problems, which should show up in the boys’ blood. Could Kara draw blood from the brothers and send it to the United States?
That was in the summer of 2011, and an uprising against dictator Moammar Gadhafi was roiling Libya. Ordinary citizens had been protesting in the streets; airports were closed. Kara was willing to help, but shipping anything out of the country was nearly impossible. And long delays in transport could ruin the blood samples.
“We were concerned that the metabolites would spoil,” Gleeson says. So his team improvised, sending instructions for an old, low-tech method of blotting drops of blood onto filter paper. Months went by. Finally, Kara emailed to say that he had given the dried blood-spot samples to a friend who was traveling out of Libya. This anonymous ally — who was rumored to be active in the anti-Gadhafi resistence, Gleeson later learned — would attempt to mail the envelope to San Diego from Europe. The U.S. researchers waited and worried.
The same risk genes can give you autism, they can give you epilepsy, or they can give you both.” Roberto Tuchman
Rare genes:
Researchers observed decades ago that seizures and autism often run hand in hand. According to a 2013 study, in the U.S., epilepsy hits around 13 percent of children with autism between ages 2 and 17, but among teenagers with autism, that rate doubles to 26 percent — far higher than the 1 percent seen in the general population. What’s more, roughly half of children with autism show unusual spikes suggestive of epilepsy on an electroencephalogram (EEG), which monitors brain activity, yet they don’t actually have epilepsy.
At first, experts thought this abnormal activity might play a role in causing autism, says neurologist Roberto Tuchman of Nicklaus Children’s Hospital in Miami, Florida, but studies haven’t borne out that idea. Instead, the bulk of the evidence seems to support the notion that common processes underpin both epilepsy and autism — and probably intellectual disability as well.
The proof for this view is largely genetic: Studies analyzing the genetics of epilepsy and of autism have pinpointed some of the same culprits. Many of these DNA defects interrupt the normal function of synapses, the junctures where neurons chatter to each other. “The same risk genes can give you autism, they can give you epilepsy, or they can give you both,” Tuchman says.
For instance, several dozen rare genetic brain disorders — some inherited, others not — are known to provoke both autism and epilepsy. In some of these conditions, such as fragile X syndrome and tuberous sclerosis, it’s obvious that the genetic snafus interfere with the brain’s early development, which can set the stage for both autism and seizures, perhaps by throwing off the normal electrical function of neural circuits. That’s further evidence that autism and epilepsy are probably “two sides of the same coin,” says Shafali Jeste, a neurologist at the University of California, Los Angeles. But even if the two conditions stem from the same biological roots, seizures may exacerbate the risk of neurodevelopmental issues in some situations, such as infantile spasms, Jeste notes; the relationship between autism and epilepsy “is a very complicated interplay.”
For Gleeson, the search into this overlap is just one quarry in a broad hunt he began in 1999 to uncover genetic causes of rare childhood brain disorders — a mission that’s sent him across the Middle East, from the United Arab Emirates to Turkey. Gene hunting in that corner of the world offers rich bounty. It has become evident in recent years that autism arises from a tangled network of hundreds of genetic factors. Teasing apart that web has been a frustrating challenge for scientists in the U.S. and the U.K. because the populations there are genetically diverse, and autism usually results from changes in multiple genes. Yet each of those changes contributes only a small bit of risk, requiring large sample sizes to detect.
But in the Middle East, it’s common for first or second cousins to wed in ‘consanguineous’ marriages, and families tend to be large. That raises the odds of spotting rare, high-impact causes of the condition: devastating ‘recessive’ genetic disorders that crop up when children inherit two copies of one mutated gene in the family bloodline. In these individuals, autism can be severe and accompanied by problems such as epilepsy and intellectual disability. By studying such cases, it’s easier to find those single-gene culprits.
Family 558:
The BCKDK detective story began in Istanbul, Turkey, with two sisters, Ceren and Seyma. Gleeson met them and the rest of ‘family 558’ in 2006 at Istanbul University’s Faculty of Medicine hospital, not far from the famed Blue Mosque.
 The hospital was equipped with the latest brain scanners and diagnostic labs, but lacked the cutting-edge genetic sequencing tools available in the U.S. The waiting room of the genetics department was crammed that day with families in line to see Gleeson, desperately seeking a full genetic evaluation of their children and their mysterious brain conditions.
The hospital was equipped with the latest brain scanners and diagnostic labs, but lacked the cutting-edge genetic sequencing tools available in the U.S. The waiting room of the genetics department was crammed that day with families in line to see Gleeson, desperately seeking a full genetic evaluation of their children and their mysterious brain conditions.
Ceren and Seyma were teenagers then. “When they came in, the girls really could not be distinguished from any other case walking in with autism,” Gleeson says. They also had intellectual disability and a history of epilepsy; both were taking epilepsy drugs.
Because Ceren and Seyma’s parents are first cousins, Gleeson hoped that the two girls might hold the key to finding what he sought — a new genetic cause for autism, and one that might also offer clues about its link to epilepsy. Working with Hülya Kayserili and Rasim Ozgur Rosti, medical geneticists at the Istanbul hospital, he took blood samples from family 558 and many others, along with copies of relevant medical records. Back in the U.S., Gleeson and his team added the samples and data to a growing database of information from hundreds of Middle Eastern families — a trove of genetic secrets waiting to be mined.
Four years later, in 2010, the team began sending hundreds of DNA samples to the Broad Institute of Harvard and MIT in Cambridge, Massachusetts, to be sequenced. At the time, a quick sequencing method that decodes only the exome (the 1 percent of the genetic blueprint that codes for proteins) offered a major advance in speed and convenience, and scientists at the Broad Institute were well equipped to handle the analysis. But the detective work only began in earnest once the San Diego group got the data back. The researchers spent countless hours filtering through all the genetic mutations and variants in the exome sequences of children with both autism and epilepsy. For each child, the researchers identified a short list of possible disease-causing variants inherited from both sides of the family tree.
The glitch in Ceren and Seyma’s BCKDK gene popped up in an analysis in early 2011 by a lab technician named Jana Schroth. What caught Schroth’s eye as she reviewed the literature was that defects in a related gene called BCKDH were well known for causing neurodevelopmental delay and seizures. That faulty gene triggers a buildup of so-called branched-chain amino acids — valine, leucine and isoleucine — which damages the brain and other organs. The result is ‘maple syrup urine disease,’ named for the odor of the affected children’s urine. The source of the amino acids is dietary protein; restricting protein in the diet eases the symptoms.
Reading up on research, Schroth saw that mutations in the BCKDK gene, located on chromosome 16, strike the same metabolic pathway, but with the opposite effect. The gene normally produces an enzyme that slows the breakdown of branched-chain amino acids; defects that disable the gene accelerate that breakdown, leaving the brain and body starved of these essential nutrients. One study had shown that deleting the BCKDK gene in mice delays their growth and causes seizures and other neurological problems.
Given all this information, the BCKDK mutations in the Turkish sisters “just screamed at us [that] this was something biologically very meaningful, and something where a new treatment could come out,” Gleeson says. In the mice, a diet rich in branched-chain amino acids corrects the growth delays. So if Ceren and Seyma’s genetic glitch were the source of their autism, epilepsy and intellectual disability, as the researchers hypothesized, the amino acids might also help treat the girls.
If their reasoning was correct, the San Diego team realized, the sisters should have a telltale sign: low blood levels of the three amino acids. Indeed, Ceren and Seyma’s parents reported that their daughters’ behavior always seemed better after they ate a meal with plenty of protein, which would deliver a big load of those amino acids. “That’s exactly what we expected,” Gleeson says. It was a hint — but it wasn’t proof.
With assistance from Kayserili in Istanbul, Gleeson’s team obtained new blood and urine samples from Ceren and Seyma, along with clinical records and videos. Previous metabolic tests had reported their blood biochemistry to be normal, but a close look at the new results showed that branched-chain amino acid levels in the sisters’ blood were well below normal. (Clinical laboratories hadn’t considered this an issue, because no one had linked it to a medical condition before.)
To confirm their findings weren’t just a fluke, the scientists needed to see more cases of people with BCKDK mutations. Gleeson and Schroth spent six months searching, reaching out to the international community of child neurologists and metabolic specialists. Had anyone else come across this problem?
“People underestimate how relevant this could be for autism as a whole.” Paul El-Fishawy
New competition:
Unbeknownst to Gleeson’s team, another group had been tracing the same research trail — but in a different country. Matthew State and his colleagues, based at Yale at the time, had also been shaking the family trees of consanguineous clans in the Middle East to find mutations that can cause neuropsychiatric conditions. In June 2009, a researcher in State’s lab named Paul El-Fishawy had visited Nagwa Meguid, founder of the autism clinic at the National Research Centre in Cairo, Egypt. They worked together to study local families, conducting autism assessments, enrolling children in the group’s study and collecting blood samples to send to the Yale lab.
Back at Yale, El-Fishawy and his colleagues sequenced these people’s exomes and zeroed in on BCKDK mutations in a 6-year-old girl and her 3-year-old brother (their parents were cousins). As with the Istanbul sisters, both the Egyptian children had classic signs of autism and had suffered from seizures as infants. The investigators also documented low levels of branched-chain amino-acids in the siblings’ blood, and began trying to find other cases. When El-Fishawy, a child psychiatrist, finally heard months later in August 2011 that Gleeson’s lab was pursuing the exact same gene, he was both elated and anxious. On the one hand, El-Fishawy says, it confirmed that he and State were on the right track. On the other, he realized that Gleeson’s team might beat them in the race to publish the research — and get the credit for discovering the mutations.
State contacted Gleeson, and the two labs agreed to join forces rather than compete. Soon afterward, Gleeson’s group wound up finding a third family — the brothers in Libya — hiding right under their noses in their own Middle East database, as they scanned through their backlog of data. The brothers had autism and intellectual disability, yet no seizures — but did they have the metabolic disorder? Results from an earlier sample were inconclusive. Only those blotter-paper samples would provide the answer.
It wasn’t until November 2011 that Kara, the neurologist in Tripoli, was able to hand off a package containing the dried-blood samples to his anonymous pal to get it to the U.S. “I think he was probably risking his life,” Gleeson says of the friend. A month later, the package finally arrived in the mail.
The samples from the Libyan boys revealed the same metabolic problem. Together, the scientists’ detective work had identified a new condition characterized by autism, epilepsy and mental impairment and caused by a rare metabolic deficiency.
Finding the three families confirmed that the team had pinpointed the correct cause of the children’s neurodevelopmental troubles — and the scientists were buoyed by the excitement they felt when “you are sure that, at that moment, you found something that is true,” says Gaia Novarino, then a postdoctoral scholar in Gleeson’s lab. “It’s a nice feeling.”
It was a striking discovery, given that the condition could be detected with a simple blood test and, in theory, had an obvious cure. Working with mice lacking BCKDK, Novarino showed that a diet enriched with branched-chain amino acids eliminates the animals’ seizures. Even more importantly, the diet also corrects certain behaviors (such as the rodents’ tendency to clasp their hind limbs together) seen in established mouse models of autism.
If the same remedy could also help people with the mutations, it would be one of the first treatments for autism. Even though BCKDK glitches are exceedingly rare, it’s possible the intervention might even be effective for children with other genetic variants: If similar biological mechanisms are involved in other forms of autism or epilepsy, “maybe we can even consider to use those amino acids to treat [those cases],” says Novarino, now at the Institute of Science and Technology in Austria.
Gleeson and State’s teams worked with their clinical colleagues overseas to test a supplement powder commonly used by bodybuilders in the U.S. that is composed of branched-chain amino acids. The investigators found that adding doses of the powder to meals several times a day brought the blood biochemistry of the Istanbul and Cairo children back to normal, just like in the mice.
The sisters in Istanbul seemed to improve for about two months, and their abnormal EEG spikes disappeared. According to Kayserili, Ceren’s parents noticed that she suddenly began speaking in full, five-word sentences rather than just using one or two words at a time. Ceren, then 21, was also calmer and less prone to angry outbursts than she had been before taking the powder. Her younger sister Seyma, then 17, seemed more attentive, alert and energetic than usual, according to the geneticist — but she still wasn’t able to put together a simple jigsaw puzzle.
The parents soon stopped the treatment, however. “They thought if there was some benefit from it, it will be very small,” says Kayserili, who now works at Koç University in Istanbul. Still, she says, supplements may have a greater effect on those with BCKDK mutations who are diagnosed and treated in infancy.
Meanwhile, the Egyptian siblings have continued taking the supplement powder. El-Fishawy and State are tracking the siblings’ progress and expect to publish results soon.
To draw firm conclusions, a proper clinical trial would also be necessary, says Gleeson. “There are so few families at this point, we don’t have really solid data,” he says. Other scientists have identified a few more people with the amino acid deficiency, including two unrelated boys in Spain. When one of the boys received six months of treatment with a branched-chain amino acid supplement, his hyperactivity and irritability improved. He had better communication and social skills, and could walk for a few seconds at a time.
Deciphering pathways:
The BCKDK story is exciting on two levels, even though few other families with this mutation have turned up. First, “the lesson we learned is that there are potentially treatable forms of autism that are hiding in the clinic,” Gleeson says. “This was just one example. But there have got to be other examples.”
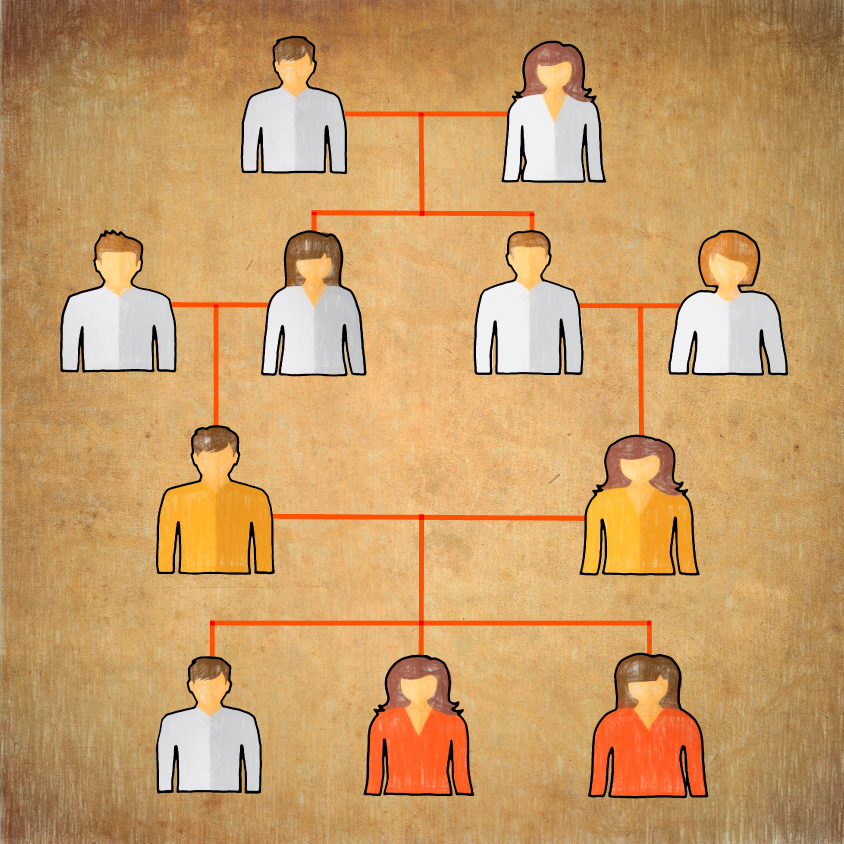
Family tree: Studying families with married cousins can help researchers spot rare disease-causing genes. In this family, two cousins (yellow) each carry a rare mutation in the BCKDK gene. Their daughters (red), with two mutated copies of the gene, have epilepsy and autism. Their son (white) has two normal copies; other forebears (white) are unknown.
Second, the newly identified metabolic disorder joins a select list of conditions associated with autism and epilepsy that each are triggered by high-impact mutations in a single gene. Although they tend to be uncommon, single-gene disorders have offered groundbreaking scientific insights, providing some of the first solid leads on potential biological mechanisms that underpin autism. “When you have a single gene completely knocked out, studying the biology is a lot easier,” El-Fishawy says, because it’s easier to see what’s broken. And, as Novarino puts it, if solving the complicated biology of all the causes of autism is like trying to read entire works of philosophy in a foreign language, then studying single-gene cases such as tuberous sclerosis and BCKDK deficiency is a Rosetta stone, deciphering mechanisms bit by bit. “We are trying to learn the alphabet,” she says. “Then maybe we are able to read back those books.”
Researchers have, for instance, learned a lot from tuberous sclerosis, a syndrome of benign tumors and developmental delay, with a high rate of both autism and epilepsy. (The syndrome can result from malfunctions in either of two genes, TSC1 or TSC2, but is considered a single-gene disorder because just one mutated gene can trigger it.) Research in mouse models of the condition showed that the problematic mutations ramp up a pathway known as mTOR. Based on these results, a drug that blocks mTOR activity won approval in 2010 for treating tumors in children with tuberous sclerosis. Other studies showed that a similar medicine alleviates seizures and cognitive problems in the mice. These findings led to the launch five years ago of the first clinical trials testing mTOR blockers for these problems in people with the syndrome.
The researchers studying BCKDK say they hope their work will likewise open doors to developing treatments for autism and epilepsy. “People underestimate how relevant this could be for autism as a whole,” El-Fishawy says. “Lots and lots of mutations and lots and lots of genes could all wind up leading to only a handful of pathways,” he says. The key is to tease apart what goes wrong in the brain when branched-chain amino acid levels are low — and find out whether the same biochemical processes are disrupted by other causes of autism and epilepsy in non-consanguineous populations in the U.S. and U.K. as well. The potential payoff from untangling these kinds of connections is big: “If we find a treatment that affects one pathway, it might be relevant to other types of genetic variants that are associated with autism spectrum disorder,” says Jeste, who is not involved in studying BCKDK.
For this next phase of the quest, Novarino and her colleagues have looked back to the mice for answers. In mice without a functional BCKDK gene, the branched-chain amino acids are low inside the brain, but other amino acids, such as phenylalanine and tyrosine, are elevated. That makes sense: These amino acids all compete with each other to cross from the blood into the brain. If branched-chain amino acids are missing, others are more likely to get in.
The resulting imbalance in amino acids may change neural activity in ways that contribute to both autism symptoms and seizures, Novarino says. For example, branched-chain amino acids are involved in making the chemical messenger glutamate, a molecule that excites brain cells, as well as GABA, a chemical messenger that inhibits neurons. Both are needed to maintain the brain’s signaling balance, and a leading theory implicates a disruption of that balance as a cause of autism. Over-excitable neurons can also prompt storms of abnormal electrical discharges in the brain, leading to seizures.
Novarino has been exploring these and other aspects in her lab, as well as studying social behaviors in the mutant mice and testing whether the supplement can change them. She is moving to publish new findings in the coming months.
The mice will prove to be a valuable model because their symptoms are so easily reversed with the supplement, Gleeson says. “Like a switch, we think you should be able to turn the autism and epilepsy on and off,” he says. “Then you can study how the circuits are changing, or you can study how the brain chemistry is changing.”
His team also plans to test the supplement’s effects on other mouse models of autism. “It’s kind of a crazy idea,” he says with a chuckle. “But I think we have to explore all possible avenues.” The amino acids have a calming effect, says Gleeson; he knows because he, along with his lab members, tried the supplement powder themselves before shipping it to the families overseas. But he doesn’t recommend that parents give the supplement to their children with autism who don’t have a mutation in BCKDK — there’s just not enough evidence that it will help, even if there are no known side effects.
In the meantime, Gleeson’s group is also working with collaborators on a study screening the exomes of 100 children in the U.S. who were diagnosed with autism before they developed seizures. As more genes are linked to both autism and epilepsy, Gleeson says, the field can make headway. “We can now start to predict which mutations or which genes are going to be associated with pure autism, or autism with epilepsy, or autism with epilepsy plus intellectual disability,” he says. That information could help prepare families and clinicians for problems to watch for, and potentially help predict which kinds of medications will work for them, he says.
The ultimate goal is to provide therapies that target the pathway that is dysfunctional in a particular set of individuals, Gleeson says. Those with deficiencies in amino acids would get one kind of treatment, for example, whereas others with, say, a defective sodium channel would get another.
A lot more scientific detective work lies ahead to nail down and characterize all the genetic culprits underlying autism and epilepsy, but the BCKDK story shows the potential rewards of gene hunting in the Middle East. Nobody knew what afflicted the children in the families with BCKDK mutations, let alone guessed that they had a metabolic disorder that might be treatable. “But now, we finally understand the cause,” Gleeson says.
His lab’s Middle East database now includes more than 5,300 families, and around 10 percent of the families have children with autism.
Twice a year, Gleeson still embarks on intense two-week trips through multiple Middle Eastern cities to recruit new families. On a trip to Libya in September 2013, he had a chance to thank the friend who smuggled out the blood samples. Gleeson and Kara had dinner with the man, who ran a chicken restaurant. “He was a kind and elderly gentleman,” Gleeson says.
Navigating through unfamiliar places, customs and languages — and political conflicts — is a challenge, but he says he loves it. “Getting there is the tough thing,” Gleeson says, “but once I’m there, the doctors and patients are really wonderful.”
By joining the discussion, you agree to our privacy policy.